medical devices guide
The Pathway from Idea to Patient under MDR
You are developing a medical device or in vitro diagnostics concept and have embarked on the exciting journey to bring your product to the patient.
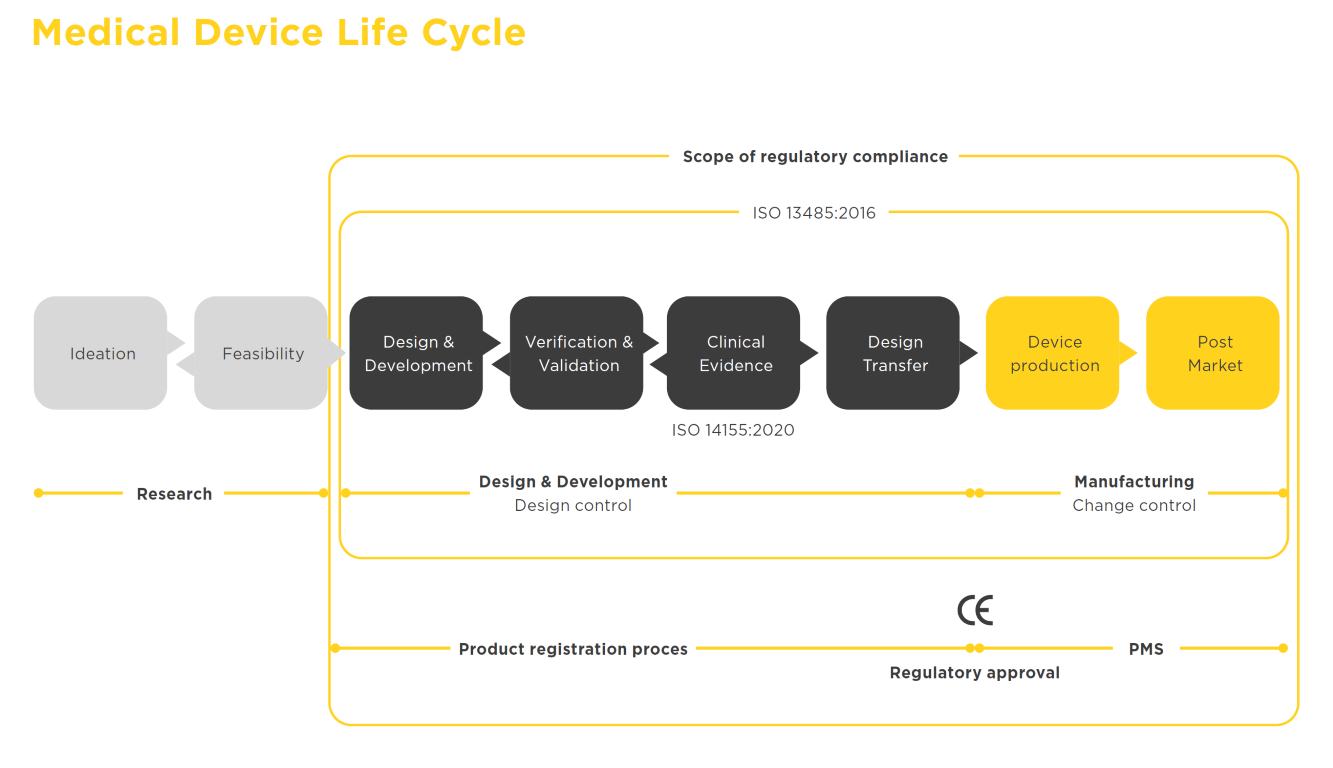
This booklet gives an overview of the pathway to follow and describes in detail the steps needed to test your device in human subjects, with the ultimate goal of bringing your product to the market.

Download now
We cover the full Medical Device product lifecycle
Topics we cover
- Business plan
- Intended use
- Device classificaion
- Technical documentation
- Quality Management System
- Conformity assessment
- Notified Bodies
- Device safety
- Clinical evidence
- Marketing authorization
- Market access
- Post-marketing
- List of abbreviations